The conversion of carbon dioxide (CO2) into carbon monoxide (CO), a key reaction in the development of synthetic fuels, can be achieved electrochemically using molecular catalysts. While the first generations of such catalysts generally relied on noble metals as active centers, a wide variety of alternatives based on abundant non-noble metals have since emerged. However, these molecular complexes suffer from limitations that prevent them from being used on a larger scale. The deployment of these systems now relies, among other things, on the possibility of immobilizing them on conductive supports for integration in electrolyzers.
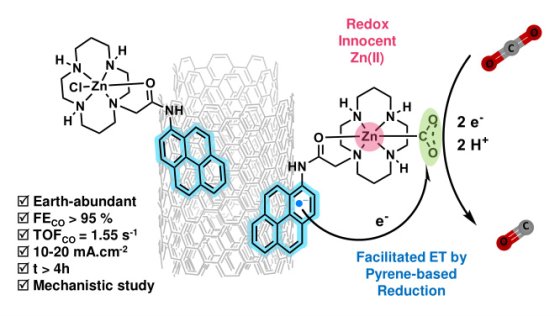
With this in mind, in an article published on September 10, 2025 in the journal ACS Catalysis, Prof. Marc Fontecave's team demonstrates the unsuspected possibility of using Zinc-based molecular complexes, a metal generally excluded due to its redox inertia. This new complex immobilized on carbon nanotubes leads to a highly active and selective electrode for the conversion ofCO2 to CO. It combines a pyrene moiety, introduced as a simple synthetic grafting tool, playing an unexpected role in electron transfer, and a zinc ion that ensures the binding and activation ofCO2 molecules, as demonstrated by computational approaches.
Sophia Ben Ahmed, Jules Sargueil, Albert Solé-Daura, Jérémy Forté, Michael Walls, Nicolas Menguy, Philipp Gotico, Ngoc Tran Huan, Yun Li, Marc Fontecave. Pyrene as a Redox Partner forCO2 Electroreduction Catalyzed by a Zinc Cyclam-Pyrene Complex. ACS Catal. 2025, 15 (18), 16439-16448. https://doi.org/10.1021/acscatal.5c03305.