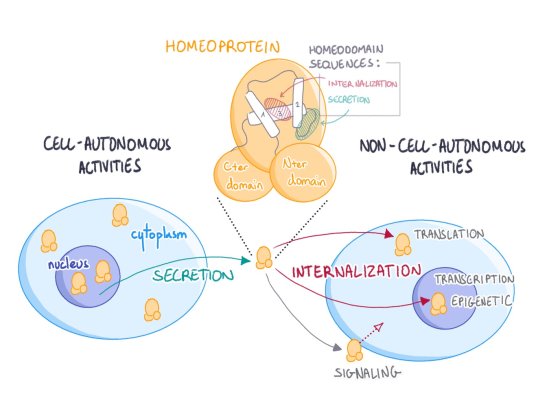
Our research is centered on the physiological functions of homeoprotein intercellular transfer, a novel signaling mechanism discovered by our group. We are using molecular and genetic approaches to explore basic aspects of these signaling transcription factors.
Brain plasticity critical periods
During infancy, childhood, and adolescence, experience and environment shape neural circuits that form the basis of adult perception and behavior. Brain circuits adapt in function of sensory, motor and social stimulation during cascading critical periods of heightened plasticity after which circuitry becomes consolidated. A number of neurodevelopmental disorders, including amblyopia, autism spectrum disorders, and schizophrenia, have now been linked to abnormalities in such experience-driven brain pathways.
The OTX2 homeoprotein regulates the opening and closure of critical periods within visual, auditory, and prefrontal cortices. OTX2 is secreted by the choroid plexus into the cerebrospinal fluid and is transferred specifically into parvalbumin (PV) GABAergic interneurons across cortices. These PV cells drive critical period onset and participate in the consolidation of circuitry. We are pursuing several projects at the epigenetic, transcriptional, and behavioral level in mice to understand how OTX2 regulates PV cell activity and how early-life stress affects the quality of critical periods. We are also studying how OTX2 is secreted by the choroid plexus and how it interacts with extracellular proteoglycans that likely play a role in targeting OTX2 to specific neurons.
Parkinson’s disease
The degeneration of dopaminergic neurons causes motor symptoms in Parkinson’s disease. Emerging evidence suggests that modulating interneurons in the primary motor cortex could offer a means to mitigate symptoms. We use acute and chronic mouse models to investigate how the induction of plasticity in the motor cortex enables local circuits adapt to the loss of dopaminergic inputs, resulting in improved motor behavior.
Amyotrophic Lateral Sclerosis
The degeneration of motoneurons in the spinal cord and the disruption of motoneuron activity in the brain occurs in Amyotrophic Lateral Sclerosis (ALS). The EN1 homeoprotein is expressed by spinal cord interneurons and is transferred into motoneurons. We have demonstrated at EN1 acts as a "rejuvenating" protein, with therapeutic action in vivo in mice and in vitro on motor neurons generated from ALS patients. Using mouse models and patient-derived iPSCs, we are investigating the molecular mechanisms (epigenetic marks, mobile genetic elements, gene expression) behind EN1-dependent neuron survival.