Our laboratory studies vascular and neuronal development, with particular interests in mechanisms that direct patterning and guidance. Specialized endothelial cells (EC) called tip cells located at the extremities of growing capillary sprouts mediate guided vascular patterning. Tip cells exhibit characteristic features, including extension of filopodia that explore the tip cell environment, lack of a lumen and a slow proliferation rate. Capillary sprouting shows morphological similarities to axon guidance. Our research is focused on the relationship between vascular and nervous system and how they develop interactions at a molecular. This approach led us to investigate how neuro-vascular interactions become functional and how deregulation of each component, vascular or neuronal, could affect the organism in health and diseases.
Our reasearch program aims to decipher neurovascular interactions in mice, molecular determinant regulating those interactions, and how neuro-vascular development can affect physiological functions. Neuronal and vascular developments require a refined spatio-temporal orchestration to guide axons and vascular plexus through the organism, align vessels with nerves, and allow appropriate functional connections to establish between the two systems. Indeed, accurate neurovascular crosstalk is of tremendous importance as it regulates many vital aspects of body homeostasis such as neuronal activity and plasticity, blood pressure and heart rate.
Very little is currently known about neurovascular development and biology, as this area of research is relatively young and requires advanced multidisciplinary knowledge. Two major axes are developed, and consist in evaluating neuronal impact onto cardio-vascular system, and vascular component within the central and peripheral nervous system.
Guidance of Angiogenesis in Peripheral Nerves:
Myelinating Schwann cells and Netrin-1 control intra-nervous vascularization of the developing mouse sciatic nerve
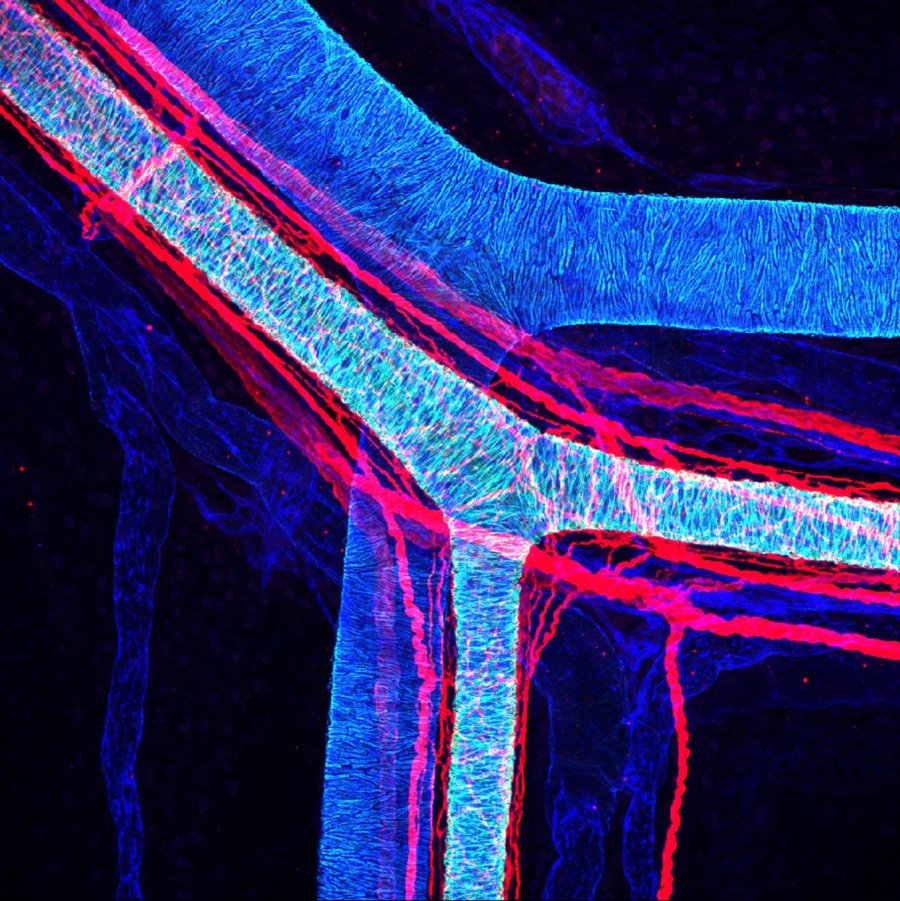
Peripheral nerves are vascularized by a dense network of blood vessels to guarantee their complex function (Figure 1). Despite the crucial role of vascularization to ensure nerve homeostasis and regeneration, the mechanisms governing nerve invasion by blood vessels remain poorly understood. We found that the sciatic nerve invasion by blood vessels begins around embryonic day 16 and continues until birth. Interestingly, intra-nervous blood vessel density significantly decreases during post-natal period, starting from P10. We show that the axon guidance molecule Netrin-1 promotes nerve invasion by blood vessels during embryogenesis. Furthermore, we identified Unc5b being specifically expressed by EC at the onset of INV whereas Netrin-1 starts at that time to be expressed at the perinerve level. Finally, myelinated Schwann cells negatively control intra-nervous vascularization during postnatal period, and regulate INV rate and morphology within the sciatic nerve. This work was done in collaboration with P Topilko (IBENS), for his expertise in myelination and Krox20 mice models 9 (Taïb S, …and Brunet I. elife 2022).
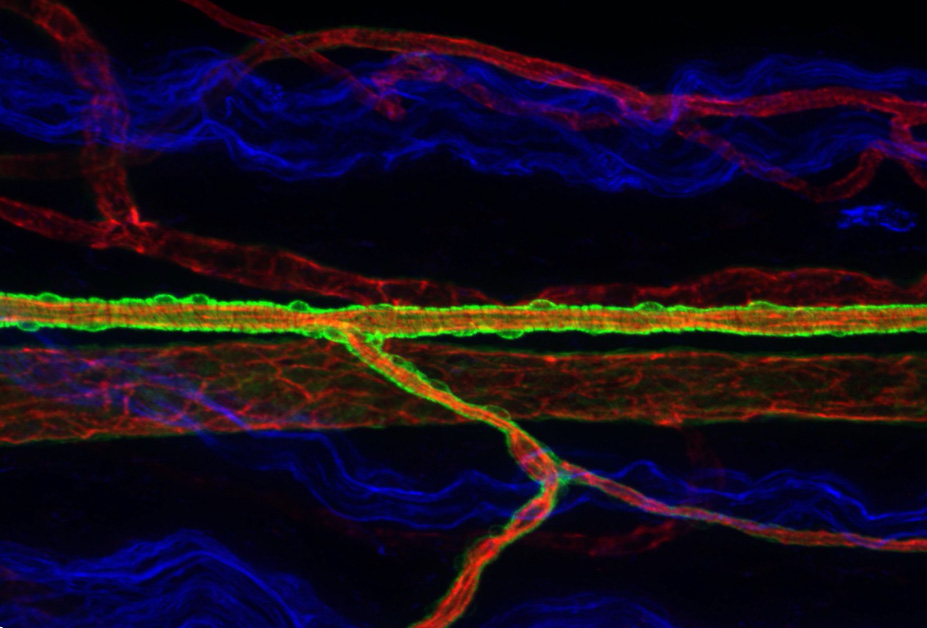
Heterogeneity and developmental dynamics of LYVE-1 perivascular macrophages distribution in the mouse brain
Brain perivascular macrophages (PVMs) are border-associated macrophages situated along blood vessels in the Virchow-Robin space and are thus found at a unique anatomical position between the endothelium and the parenchyma. Owing to their location and phagocytic capabilities, PVMs are regarded as important components that regulate various aspects of brain physiology in health and pathophysiological states. In our last study (Karam et al. JCBFM 2022), we used LYVE-1 to identify PVMs in the mouse brain using used brain-tissue sections and cleared whole-brains ( Figure 2) to learn about how they are distributed within the brain and across different developmental postnatal stages. We find that LYVE-1+ PVMs associate with the vasculature in different patterns and proportions depending on vessel diameter or arterio-venous differentiation. LYVE-1+ PVMs relate to blood vessels in a brain-region-dependent manner. We show that their postnatal distribution is developmentally dynamic and peaks at P10-P20 depending on the brain region. PVMs express CD206 (another non-lymphatic marker of PVMs) and LYVE-1 at different ratio throughout the brain suggesting that PVMs possess a unique molecular signature. We further demonstrate that their density is reduced in the APP/PS1 mouse model of Alzheimer’s Disease inversely proportional to beta-amyloid deposits. Interestingly, we found that PVMs conserve CD206 but not LYVE-1 expression in APP/PS1 compared to WT, thus losing their lymphatic and possibly drainage abilitites.
In conclusion, our results reveal unexpected heterogeneity and dynamics of LYVE-1+ PVMs, with selective coverage in brain vasculature, compatible with potential unexplored roles for this population of PVMs in postnatal development, and in regulating brain functions in steady-state and disease conditions.
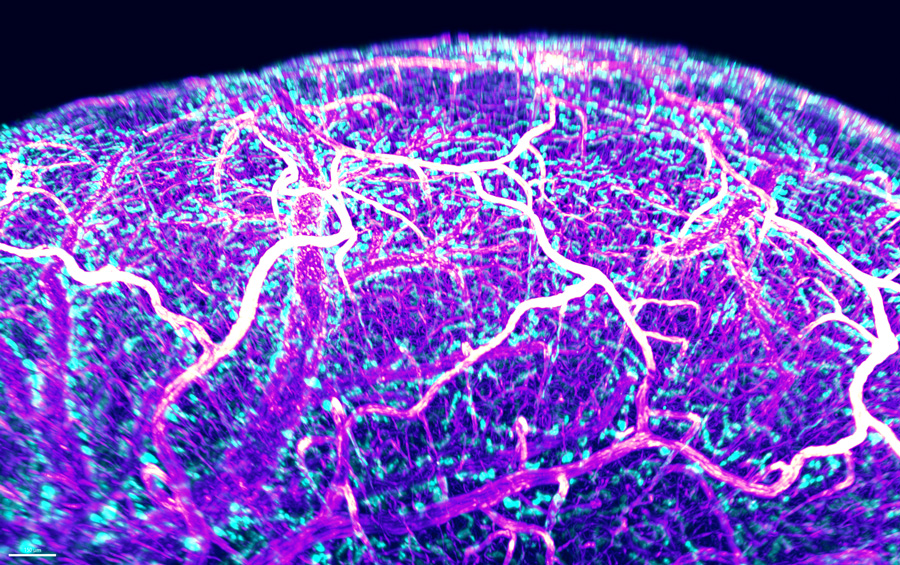
Sympathetic cardiovascular Innervation
Our as yet unpublished work (Simonnet. Martin et al.) directly investigates interactions between the nervous and the vascular systems, in particular arterial innervation (Figure 3). Innervation of peripheral resistance arteries by autonomic sympathetic nerves controls blood supply to organs by regulating vascular tone. Despite the fundamental importance of blood flow control and vascular tone, signals controlling the development of sympathetic arterial innervation are currently unknown. We have determined the developmental time window when arterial innervation is initiated in mice, and identified several novel regulators of arterial innervation that we are currently characterizing. Deletion of those regulators in mice models in a cell-type and inducible fashion give rise to hypo or hyper-innervation and we have now models allowing to modifie sympathetic innervation to study the impact of arterial innervation in physiological and pathological conditions such as hypertension.