Virginijus Šikšnys est invité par l'assemblée du Collège de France sur proposition de la Pr Edith Heard.
Résumé
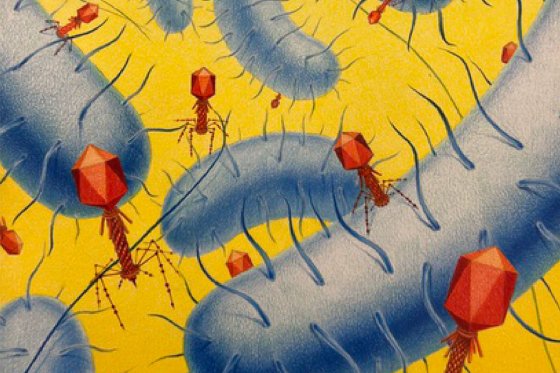
Bacteriophages (viruses that infect bacteria) pose a lethal threat to bacteria. In response, bacteria evolved multiple defense barriers that comprise a primitive immune system of bacteria. CRISPR-Cas system functions as an adaptive immune system that provides resistance against invading viruses. CRISPR-Cas immunity is enabled by programmable nucleases that act as DNA scissors that recognize and destroy invading nucleic acids. Easy programmability of CRISPR-Cas nucleases paved the way for the development of versatile tools for targeted genome engineering. Currently, Cas9 and Cas12 CRISPR-Cas nucleases are rapidly advancing into the clinics for the treatment of different diseases. Despite this exciting progress, significant challenges remain and need to be overcome, including precise delivery to target tissues, safety related to off-target effects and immune interactions and low efficiency of gene insertions. Therefore, in order to optimize therapeutic potential of genome editing technologies, novel tools that are more compact, more precise and safer are highly desirable. Recently, very compact RNA-directed transposon-related TnpB nucleases have been identified enabling the development of a new class of DNA-scissors. Further exploration and systematic discovery of novel antiviral defense systems is likely to decode novel enzymatic functions that could be repurposed for technological applications.
