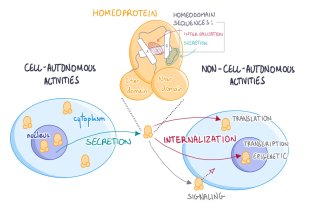
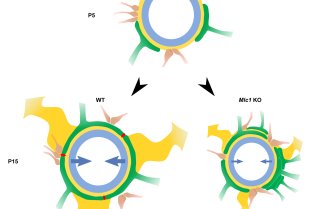
CNRS/UMR 7241 – Inserm U1050
Le Centre interdisciplinaire de recherche en biologie (CIRB), créé en 2009 à l’initiative d’Alain Prochiantz, est situé sur le site Marcelin-Berthelot du Collège de France. Le CIRB est actuellement composé de vingt et une équipes, dont les thématiques de recherche couvrent aussi bien la microbiologie, la biologie cellulaire, la biologie du développement, les neurosciences et l’évolution que la biologie du cancer.
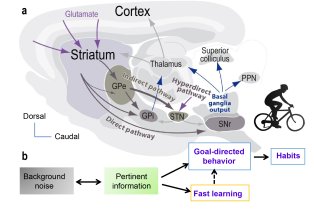
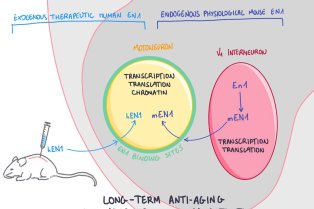
Le CIRB offre un environnement intégré et original pour mener des recherches sur des questions pointues de la biologie moderne. Notre force et notre originalité résident dans l’association de scientifiques ayant des cultures et des expertises différentes. Les membres du CIRB développent à la fois des approches expérimentales et théoriques et sont intéressés par les mécanismes fondamentaux qui sous-tendent les processus biologiques.